Write the equation for the combustion reaction of benzene. Calculation of parameters of benzene combustion products in air. Alkylation of aromatic hydrocarbons
The first group of reactions are substitution reactions. We said that arenes do not have multiple bonds in the structure of the molecule, but contain a conjugated system of six electrons, which is very stable and gives additional strength to the benzene ring. Therefore, in chemical reactions, the replacement of hydrogen atoms occurs first, and not the destruction of the benzene ring.
We have already encountered substitution reactions when talking about alkanes, but for them these reactions followed a radical mechanism, while arenes are characterized by an ionic mechanism of substitution reactions.
First chemical property halogenation. Replacement of a hydrogen atom with a halogen atom, chlorine or bromine.
The reaction occurs when heated and always with the participation of a catalyst. In the case of chlorine, it could be aluminum chloride or ferric chloride three. The catalyst polarizes the halogen molecule, causing heterolytic bond cleavage and producing ions.
Chlorine is a positively charged ion and reacts with benzene.
If the reaction occurs with bromine, then the catalyst is iron bromide or aluminum bromide.

It is important to note that the reaction occurs with molecular bromine and not with bromine water. Benzene does not react with bromine water.
The halogenation of benzene homologues has its own characteristics. In the toluene molecule, the methyl group facilitates substitution in the ring, reactivity increases, and the reaction occurs under milder conditions, that is, at room temperature.

It is important to note that substitution always occurs in the ortho and para positions, so a mixture of isomers is obtained.
Second property nitration of benzene, introduction of a nitro group into the benzene ring.

A heavy yellowish liquid with the smell of bitter almonds is formed nitrobenzene, so the reaction can be qualitative to benzene. For nitration, a nitrating mixture of concentrated nitric and sulfuric acids is used. The reaction is carried out by heating.
Let me remind you that for the nitration of alkanes in the Konovalov reaction, diluted nitric acid was used without the addition of sulfuric acid.
During the nitration of toluene, as well as during halogenation, a mixture of ortho- and para-isomers is formed.

Third property alkylation of benzene with haloalkanes.
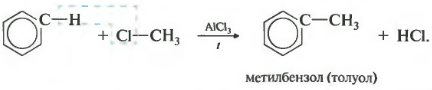
This reaction allows the introduction of a hydrocarbon radical into the benzene ring and can be considered a method for producing benzene homologues. Aluminum chloride is used as a catalyst, which promotes the decomposition of the haloalkane molecule into ions. Heating is also necessary.
Fourth property alkylation of benzene with alkenes.

In this way you can obtain, for example, cumene or ethylbenzene. Catalyst aluminum chloride.
2. Addition reactions to benzene
The second group of reactions are addition reactions. We said that these reactions are not typical, but they are possible under fairly stringent conditions with the destruction of the pi-electron cloud and the formation of six sigma bonds.
Fifth property in the general list hydrogenation, addition of hydrogen.
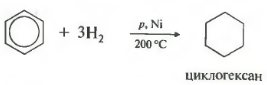
Temperature, pressure, catalyst nickel or platinum. Toluene can react in the same way.
Sixth property chlorination. Please note that we are talking specifically about interaction with chlorine, since bromine does not enter into this reaction.

The reaction occurs under harsh ultraviolet irradiation. Hexachlorocyclohexane, another name for hexachlorane, a solid, is formed.
It is important to remember that for benzene not possible addition reactions of hydrogen halides (hydrohalogenation) and addition of water (hydration).
3. Substitution in the side chain of benzene homologues
The third group of reactions concerns only benzene homologues - this is a substitution in the side chain.
Seventh property in the general list halogenation at the alpha carbon atom in the side chain.
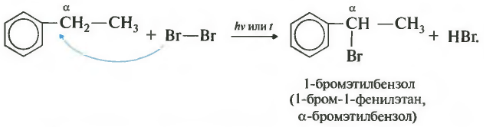
The reaction occurs when heated or irradiated and always only at the alpha carbon. As halogenation continues, the second halogen atom will return to the alpha position.
4. Oxidation of benzene homologues
The fourth group of reactions is oxidation.
The benzene ring is too strong, so benzene does not oxidize potassium permanganate does not discolor its solution. This is very important to remember.
But benzene homologues are oxidized by an acidified solution of potassium permanganate when heated. And this is the eighth chemical property.
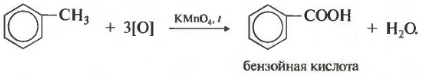
This produces benzoic acid. Discoloration of the solution is observed. In this case, no matter how long the carbon chain of the substituent is, it always breaks after the first carbon atom and the alpha atom is oxidized to a carboxyl group with the formation of benzoic acid. The remainder of the molecule is oxidized to the corresponding acid or, if it is only one carbon atom, to carbon dioxide.
If a benzene homologue has more than one hydrocarbon substituent on the aromatic ring, then oxidation occurs according to the same rules - the carbon located in the alpha position is oxidized.
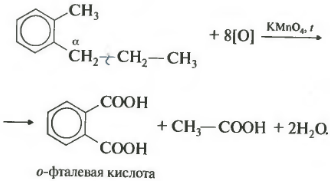
This example produces a dibasic aromatic acid called phthalic acid.
I would especially like to note the oxidation of cumene, isopropylbenzene, by atmospheric oxygen in the presence of sulfuric acid.
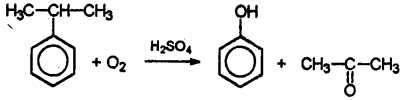
This is the so-called cumene method for producing phenol. As a rule, one encounters this reaction in matters related to the production of phenol. This is an industrial method.
Ninth property combustion, complete oxidation with oxygen. Benzene and its homologues burn to carbon dioxide and water.
Let us write the combustion equation of benzene in general form.
According to the law of conservation of mass, there should be as many atoms on the left as there are atoms on the right. Because in chemical reactions, atoms do not disappear, but the order of bonds between them simply changes. So there will be as many carbon dioxide molecules as there are carbon atoms in the arene molecule, since the molecule contains one carbon atom. That is, n CO 2 molecules. There will be two times fewer water molecules than hydrogen atoms, that is (2n-6)/2, which means n-3.
There are the same number of oxygen atoms on the left and right. On the right there are 2n from carbon dioxide, because each molecule has two oxygen atoms, plus n-3 from water, for a total of 3n-3. On the left there are the same number of oxygen atoms 3n-3, which means there are two times fewer molecules, because the molecule contains two atoms. That is (3n-3)/2 oxygen molecules.
Thus, we have compiled an equation for the combustion of benzene homologues in general form.
Aromatic HCs (arenas)– these are hydrocarbons whose molecules contain one or more benzene rings.
Examples of aromatic hydrocarbons:

Arenas of the benzene series (monocyclic arenes)
General formula:C n H 2n-6 , n≥6
The simplest representative of aromatic hydrocarbons is benzene, its empirical formula is C 6 H 6.
Electronic structure of the benzene molecule
The general formula of monocyclic arenes C n H 2 n -6 shows that they are unsaturated compounds.
In 1856, the German chemist A.F. Kekule proposed a cyclic formula for benzene with conjugated bonds (single and double bonds alternate) - cyclohexatriene-1,3,5: 
This structure of the benzene molecule did not explain many of the properties of benzene:
- benzene is characterized by substitution reactions rather than addition reactions characteristic of unsaturated compounds. Addition reactions are possible, but are more difficult than for ;
- benzene does not enter into reactions that are qualitative reactions to unsaturated hydrocarbons (with bromine water and KMnO 4 solution).
Later electron diffraction studies showed that all bonds between carbon atoms in a benzene molecule have the same length of 0.140 nm (the average value between the length of a simple S-S connections 0.154 nm and double bond C=C 0.134 nm). The angle between the bonds at each carbon atom is 120 o. The molecule is a regular flat hexagon.
Modern theory to explain the structure of the C 6 H 6 molecule uses the idea of hybridization of atomic orbitals.
The carbon atoms in benzene are in a state of sp 2 hybridization. Each "C" atom forms three σ bonds (two with carbon atoms and one with a hydrogen atom). All σ bonds are in the same plane:
Each carbon atom has one p-electron, which does not participate in hybridization. The unhybridized p-orbitals of carbon atoms are in a plane perpendicular to the plane of σ bonds. Each p-cloud overlaps with two neighboring p-clouds, and as a result a single conjugated π-system is formed (remember the effect of conjugation of p-electrons in the 1,3-butadiene molecule, discussed in the topic “Diene hydrocarbons”):
The combination of six σ-bonds with a single π-system is called aromatic connection.
A ring of six carbon atoms linked by an aromatic bond is called benzene ring or benzene ring.
In accordance with modern ideas about the electronic structure of benzene, the C 6 H 6 molecule is depicted as follows:

Physical properties of benzene
Benzene under normal conditions is a colorless liquid; t o pl = 5.5 o C; t o kip. = 80 o C; has a characteristic odor; does not mix with water, good solvent, highly toxic.
Chemical properties of benzene
The aromatic connection determines Chemical properties benzene and other aromatic hydrocarbons.
The 6π-electron system is more stable than ordinary two-electron π-bonds. Therefore, addition reactions are less typical for aromatic hydrocarbons than for unsaturated hydrocarbons. The most characteristic reactions for arenes are substitution reactions.
I. Substitution reactions
1.Halogenation

2. Nitration
The reaction is carried out with a mixture of acids (nitrating mixture): 
3.Sulfonation

4.Alkylation (replacement of the “H” atom with an alkyl group) – Friedel-Crafts reactions, benzene homologues are formed:

Instead of haloalkanes, alkenes can be used (in the presence of a catalyst - AlCl 3 or inorganic acid):

II. Addition reactions
1.Hydrogenation

2.Addition of chlorine

III.Oxidation reactions
1. Combustion
2C 6 H 6 + 15O 2 → 12CO 2 + 6H 2 O
2. Incomplete oxidation (KMnO 4 or K 2 Cr 2 O 7 in an acidic environment). The benzene ring is resistant to oxidizing agents. No reaction occurs.
Obtaining benzene
In industry:
1) oil and coal processing;
2) dehydrogenation of cyclohexane:

3) dehydrocyclization (aromatization) of hexane:

In the laboratory:
Fusion of benzoic acid salts with:

Isomerism and nomenclature of benzene homologues
Any homolog of benzene has a side chain, i.e. alkyl radicals bound to a benzene ring. The first benzene homologue is a benzene ring bonded to a methyl radical: 
Toluene has no isomers, since all positions in the benzene ring are equivalent.
For subsequent homologues of benzene, one type of isomerism is possible - side chain isomerism, which can be of two types:
1) isomerism of the number and structure of substituents;
2) isomerism of the position of substituents.



Physical properties of toluene
Toluene- a colorless liquid with a characteristic odor, insoluble in water, soluble in organic solvents. Toluene is less toxic than benzene.
Chemical properties of toluene
I. Substitution reactions
1.Reactions involving the benzene ring
Methylbenzene enters into all substitution reactions in which benzene is involved, and at the same time exhibits higher reactivity, reactions proceed at a faster rate.
The methyl radical contained in the toluene molecule is a substituent of the kind, therefore, as a result of substitution reactions in the benzene ring, ortho- and para-derivatives of toluene are obtained or, in case of an excess of the reagent, triderivatives of the general formula:

a) halogenation


With further chlorination, dichloromethylbenzene and trichloromethylbenzene can be obtained:

II. Addition reactions
Hydrogenation

III.Oxidation reactions
1.Combustion
C 6 H 5 CH 3 + 9O 2 → 7CO 2 + 4H 2 O
2. Incomplete oxidation
Unlike benzene, its homologues are oxidized by certain oxidizing agents; in this case, the side chain is subject to oxidation, in the case of toluene, the methyl group. Mild oxidizing agents such as MnO 2 oxidize it to an aldehyde group, stronger oxidizing agents (KMnO 4) cause further oxidation to an acid: 
Any homologue of benzene with one side chain is oxidized by a strong oxidizing agent such as KMnO4 into benzoic acid, i.e. the side chain breaks with oxidation of the split-off part to CO 2; For example: 
If there are several side chains, each of them is oxidized to a carboxyl group and as a result polybasic acids are formed, for example:

Obtaining toluene:
In industry:
1) oil and coal processing;
2) dehydrogenation of methylcyclohexane:

3) dehydrocyclization of heptane:
In the laboratory:
1) Friedel-Crafts alkylation;
2) Wurtz-Fittig reaction(reaction of sodium with a mixture of halobenzene and haloalkane).
Arenas (aromatic hydrocarbons) – these are unsaturated (unsaturated) cyclic hydrocarbons, the molecules of which contain stable cyclic groups of atoms (benzene nuclei) with a closed system of conjugated bonds.
General formula: C n H 2n–6for n ≥ 6.
Chemical properties of arenes
Arenas– unsaturated hydrocarbons, the molecules of which contain three double bonds and a ring. But due to the conjugation effect, the properties of arenes differ from the properties of other unsaturated hydrocarbons.
Aromatic hydrocarbons are characterized by the following reactions:
- accessions,
- substitutions,
- oxidation (for benzene homologues).
The aromatic system of benzene is resistant to oxidizing agents. However, benzene homologues are oxidized by potassium permanganate and other oxidizing agents.
1. Addition reactions
Benzene adds chlorine in the light and hydrogen when heated in the presence of a catalyst.
1.1. Hydrogenation
Benzene adds hydrogen when heated and under pressure in the presence of metal catalysts (Ni, Pt, etc.).
When benzene is hydrogenated, cyclohexane is formed:
When homologs are hydrogenated, cycloalkane derivatives are formed. When toluene is heated with hydrogen under pressure and in the presence of a catalyst, methylcyclohexane is formed:
1.2. Chlorination of arenas
The addition of chlorine to benzene occurs by radical mechanism with high temperature , under the influence of ultraviolet radiation.
When benzene is chlorinated in light, it forms 1,2,3,4,5,6-hexachlorocyclohexane (hexachlorane).
Hexachlorane is a pesticide used to control harmful insects. The use of hexachlorane is currently prohibited.
Benzene homologues do not add chlorine. If a benzene homolog reacts with chlorine or bromine in light or at high temperature (300°C), then hydrogen atoms are replaced on the pendant alkyl substituent rather than on the aromatic ring.
2. Substitution reactions
2.1. Halogenation
Benzene and its homologues enter into substitution reactions with halogens (chlorine, bromine) in the presence of catalysts (AlCl 3, FeBr 3) .
When interacting with chlorine on the AlCl 3 catalyst, chlorobenzene is formed:
Aromatic hydrocarbons react with bromine when heated and in the presence of a catalyst - FeBr 3. Metallic iron can also be used as a catalyst.
Bromine reacts with iron to form iron(III) bromide, which catalyzes the bromination of benzene:
Meta-chlorotoluene is formed in small quantities.
When benzene homologues interact with halogens in light or at high temperatures(300 o C) hydrogen is replaced not in the benzene ring, but in the side hydrocarbon radical.
For example, when chlorinating ethylbenzene:
2.2. Nitration
Benzene reacts with concentrated nitric acid in the presence of concentrated sulfuric acid (nitrating mixture).
This produces nitrobenzene:
Toluene reacts with concentrated nitric acid in the presence of concentrated sulfuric acid.
In the reaction products we indicate either O-nitrotoluene:
or P-nitrotoluene:
Nitration of toluene can also occur with the replacement of three hydrogen atoms. This produces 2,4,6-trinitrotoluene (TNT, tol):
2.3. Alkylation of aromatic hydrocarbons
- Arenes react with haloalkanes in the presence of catalysts (AlCl 3, FeBr 3, etc.) to form benzene homologues.
- Aromatic hydrocarbons react with alkenes in the presence of aluminum chloride, iron (III) bromide, phosphoric acid, etc.
- Alkylation with alcohols occurs in the presence of concentrated sulfuric acid.
2.4. Sulfonation of aromatic hydrocarbons
Benzene reacts when heated with concentrated sulfuric acid or a solution of SO 3 in sulfuric acid (oleum) to form benzenesulfonic acid:
3. Oxidation of arenes
Benzene is resistant to even strong oxidizing agents. But benzene homologues are oxidized under the influence of strong oxidizing agents. Benzene and its homologues burn.
3.1. Complete oxidation - combustion
When benzene and its homologues burn, carbon dioxide and water are formed. The combustion reaction of arenes is accompanied by the release of a large amount of heat.
2C 6 H 6 + 15O 2 → 12CO 2 + 6H 2 O + Q
The combustion equation of arenes in general form:
C n H 2n–6 + (3n – 3)/2 O 2 → nCO 2 + (n – 3)H 2 O + Q
When aromatic hydrocarbons burn in a lack of oxygen, carbon monoxide CO or soot C can be formed.
Benzene and its homologues burn in air with a smoky flame. Benzene and its homologues form explosive mixtures with air and oxygen.
3.2. ABOUToxidation of benzene homologues
Benzene homologues are easily oxidized by potassium permanganate and dichromate in an acidic or neutral environment when heated.
This happens oxidation of all bonds at a carbon atom, adjacent to the benzene ring, except for the bond of this carbon atom with the benzene ring.
Toluene oxidizes potassium permanganate in sulfuric acid with education benzoic acid:
If toluene oxidation occurs in a neutral solution when heated, then it is formed benzoic acid salt - potassium benzoate:
Thus, toluene decolorizes an acidified solution of potassium permanganate when heated.
Longer radicals are oxidized to benzoic acid and carboxylic acid:
The oxidation of propylbenzene produces benzoic and acetic acids:
Isopropylbenzene is oxidized by potassium permanganate in an acidic environment to benzoic acid and carbon dioxide:
4. Orienting effect of substituents on the benzene ring
If the benzene ring contains substituents, not only alkyl, but also containing other atoms (hydroxyl, amino group, nitro group, etc.), then the substitution reactions of hydrogen atoms in the aromatic system proceed in a strictly defined manner, in accordance with the nature influence of the substituent on the aromatic π-system.
Types of substituents on the benzene ring
| Substituents of the first kind | Substituents of the second kind |
| ortho- And pair-position | Further replacement occurs mainly in meta-position |
| Electron donor, increases electron density in the benzene ring | Electron-withdrawing, they reduce the electron density in the conjugated system. |
|
|
We present to your attention a video lesson dedicated to the topic “Chemical properties of benzene”. Using this video, you can gain an understanding of the chemical properties of benzene, as well as the harsh conditions required for benzene to react with other substances.
Subject:Aromatic hydrocarbons
Lesson:Chemical properties of benzene
Rice. 1. Benzene molecule
It is difficult to break the p-electron cloud in a benzene molecule. Therefore, benzene enters into chemical reactions much less actively compared to unsaturated compounds.


In order for benzene to enter into chemical reactions, fairly stringent conditions are required: elevated temperature, and in many cases, a catalyst. In most reactions, the stable benzene ring is retained.
1. Bromination.

A catalyst (iron (III) or aluminum bromide) is required and even small amounts of water are not allowed. The role of the catalyst is that the bromine molecule is attracted by one of the bromine atoms to the iron atom. As a result, it is polarized - a pair of bonding electrons goes to the bromine atom associated with iron:
Br +…. Br - FeBr 3 .
Br+ is a strong electrophile. It is attracted to the six-electron cloud of the benzene ring and breaks it, forming a covalent bond with the carbon atom:
A bromine anion could join the resulting cation. But the reduction of the aromatic system of the benzene ring is energetically more favorable than the addition of the bromine anion. Therefore, the molecule goes into a stable state, throwing out a hydrogen ion:
All electrophilic substitution reactions in the benzene ring proceed by a similar mechanism.
2. Nitration

Benzene and its homologues react with a mixture of concentrated sulfuric and nitric acids (nitrating mixture). In the nitrating mixture, the nitronium ion NO 2 + exists in equilibrium, which is an electrophile:

3. Sulfonation.
Benzene and other arenes, when heated, react with concentrated sulfuric acid or oleum - a solution of SO 3 in sulfuric acid:

4 . Friedel-Crafts alkylation
5. Alkylation with alkenes

These reactions are energetically unfavorable and therefore only occur when heated or irradiated.
1. Hydrogenation.
When heated, under elevated pressure, and in the presence of a Ni, Pt, or Pd catalyst, benzene and other arenes add hydrogen to form cyclohexane:

2. Chlorination of benzene.
Under the influence of ultraviolet radiation, benzene adds chlorine. If a quartz glass flask containing a solution of chlorine in benzene is exposed to sunlight, the solution will quickly become discolored and the chlorine will combine with benzene to form 1,2,3,4,5,6-hexachlorocyclohexane, which is known as hexachlorane(previously used as an insecticide):

3. Benzene combustion.
Unlike alkanes, benzene and other aromatic hydrocarbons have a bright, smoky flame.
Summing up the lesson
In this lesson you studied the topic “Chemical properties of benzene”. Using this material, you were able to gain an understanding of the chemical properties of benzene, as well as the harsh conditions that are necessary for benzene to react with other substances.
Bibliography
1. Rudzitis G.E. Chemistry. Fundamentals of general chemistry. 10th grade: textbook for general education institutions: a basic level of/ G. E. Rudzitis, F. G. Feldman. - 14th edition. - M.: Education, 2012.
2. Chemistry. Grade 10. Profile level: textbook for general education institutions/ V.V. Eremin, N.E. Kuzmenko, V.V. Lunin et al. - M.: Bustard, 2008. - 463 p.
3. Chemistry. Grade 11. Profile level: academic. for general education institutions/ V.V. Eremin, N.E. Kuzmenko, V.V. Lunin et al. - M.: Bustard, 2010. - 462 p.
4. Khomchenko G.P., Khomchenko I.G. Collection of problems in chemistry for those entering universities. - 4th ed. - M.: RIA "New Wave": Publisher Umerenkov, 2012. - 278 p.
Homework
1. Nos. 13, 14 (p. 62) Rudzitis G.E., Feldman F.G. Chemistry: Organic chemistry. 10th grade: textbook for general education institutions: basic level / G. E. Rudzitis, F.G. Feldman. - 14th edition. - M.: Education, 2012.
2. Why do aromatic compounds differ in chemical properties from both saturated and unsaturated hydrocarbons?
3. Write the reaction equations for the combustion of ethylbenzene and xylene.
Read also...
- Motivational theories. Motive and motivation. Theories of motivation Theories of motivation in various psychological directions
- Purpose of the Phillips School Anxiety Test
- Samara State Regional Academy
- M. V. Koltunova language and business communication. Language and business communication Etiquette and protocol of business communication